Top Medical Body Slams Study On Covaxin Safety, Side-Effects, Wants Apology
Bharat Biotech’s Covaxin was one of two Covid-19 vaccines available in India (File).
New Delhi:
The Indian Council of Medical Research, or ICMR, has distanced itself from a follow-up study by two Banaras Hindu University professors that said nearly a third of 926 individuals who received the India-made Covaxin COVID-19 vaccine reported serious side-effects.
The study claimed that around one per cent of respondents reported strokes and an autoimmune disorder called Guillain-Barre Syndrome, which causes weakness in nerves in the arms and legs.
Conducted between January 2022 and August 2023, the study also said 50 per cent of the sample size complained of respiratory infections, and over 30 per cent reported assorted physical problems, ranging from skin and nervous system disorders to bone and muscle problems.
READ | Over 30% Covaxin Takers Had Health Issues After Year: Study
Specifically, between adolescents and adults respondents the study claimed 10.5 per cent reported new-onset skin and subcutaneous disorders and 10.2 per cent claimed nervous system concerns.
Among female respondents, 4.6 per cent claimed menstrual disorders.
ICMR Criticises Study Flagging Covaxin AESIs
However, the ICMR faulted the study – ‘Long-Term Safety Analysis of BBVl52 Coronavirus Vaccine in Adolescents and Adults: Findings From a 1-Year Prospective Study in North India’ published by Springer Nature – for poor methodology, and objected to it “acknowledging” the medical body.
ICMR Director-General Rajiv Bahl said the study had no control arm (of unvaccinated individuals) to compare rate of AESIs, or Adverse Events of Special Interest, and, therefore, the reported side-effects could not be linked or attributed to being administered Covaxin as COVID-19 vaccination.
Continuing a long list of critical observations, the ICMR boss also declared the study does not provide background rates of observed events in the population, making it impossible to assess change in such incidences in the post-vaccination period.
FACT CHECK | Can Covaxin Lead To Death After 2 Years Of Vaccination?
The method of data collection – study participants were contacted via telephone a year after vaccination and their responses were recorded without confirming them via medical records or examination by doctors – was also severely criticised.
Dr Bahl stressed that the ICMR is not associated with the study claiming side-effects from the India-developed vaccine, and had not provided any support – technical or financial – to the study authors.
The study authors and publishers have been urged to remove the acknowledgement to the ICMR.
“The authors are urged to rectify acknowledgment to ICMR and publish an erratum. Additionally, they are asked to address methodological concerns raised,” the ICMR chief said.
“Failure to do so may prompt ICMR to consider legal and administrative action.”
What Study Authors Said
Among the points made, the researchers acknowledged that understanding the AESIs-vaccines link required a control arm of unvaccinated individuals to compare rates of reported serious side-effects.
The authors also said that in the absence of data on background rates of observed AESIs, no comments were possible on changes in incidence of observed events in post-vaccination periods.
“Our findings are confined to BBV152 (Covaxin) and should not be extrapolated to viral vector or mRNA vaccines. The study primarily involved adolescents and the sample size of adults was relatively small. Larger adult-based studies are needed to understand long-term safety of BBV152 in adults.”
Bharat Biotech Responds
The study has also been questioned by Covaxin developers and manufacturers Bharat Biotech, which pointed to multiple alternate studies to emphasise its drug’s “excellent safety track record”.
READ | Bharat Biotech Flags Covaxin’s Excellent Safety Record In Risks Row
The ICMR’s strong reaction to the study comes amid concern over potentially fatal side-effects for those who received the vaccine developed by British pharma giant AstraZeneca and Oxford University, and manufactured and sold in India – as Covishield – by the Serum Institute.
Concerns Over Covishield
Given the questions about Covishield and Covaxin’s long-term safety records, a group of doctors last week called for a review all the science behind all Covid vaccines, as well implementation of surveillance and monitoring measures to ensure vaccine AESIs are identified as early as possible.
READ | Covishield Safety Row: Doctors Urge Centre To Review All Vaccines
The spotlight, so far, has been squarely on Covishield after AstraZeneca was hit by multiple lawsuits in the United Kingdom, with at least one patient claiming he suffered a serious brain injury following a blood clot that formed after he was injected in April 2021.
Fact Check | Are Indians Who Got Covishield Vaccine Susceptible To TTS?
AstraZeneca initially contested the claim but later said its vaccine could “in very rare cases” cause Thrombosis with Thrombocytopenia Syndrome, or TTS, which is a medical condition that can cause blood clots and low platelet counts, and has been linked to over 80 deaths in the UK alone.
READ | AstraZeneca Drops Covid Vaccine After Rare Side Effect Report
The company has expressed sympathy for those who have lost loved ones or reported health problems, but has reiterated its commitment to patient safety and adherence to “stringent (safety) standards”
It has also announced a global withdrawal of all vaccine stock, but attributed the withdrawal to commercial reasons, i.e., to a “surplus of available updated vaccines” for COVID-19.
Vaccinations In India
As of this morning, approximately 17 per cent of all vaccines administered were Covaxin, according to data from the government’s CoWIN dashboard.
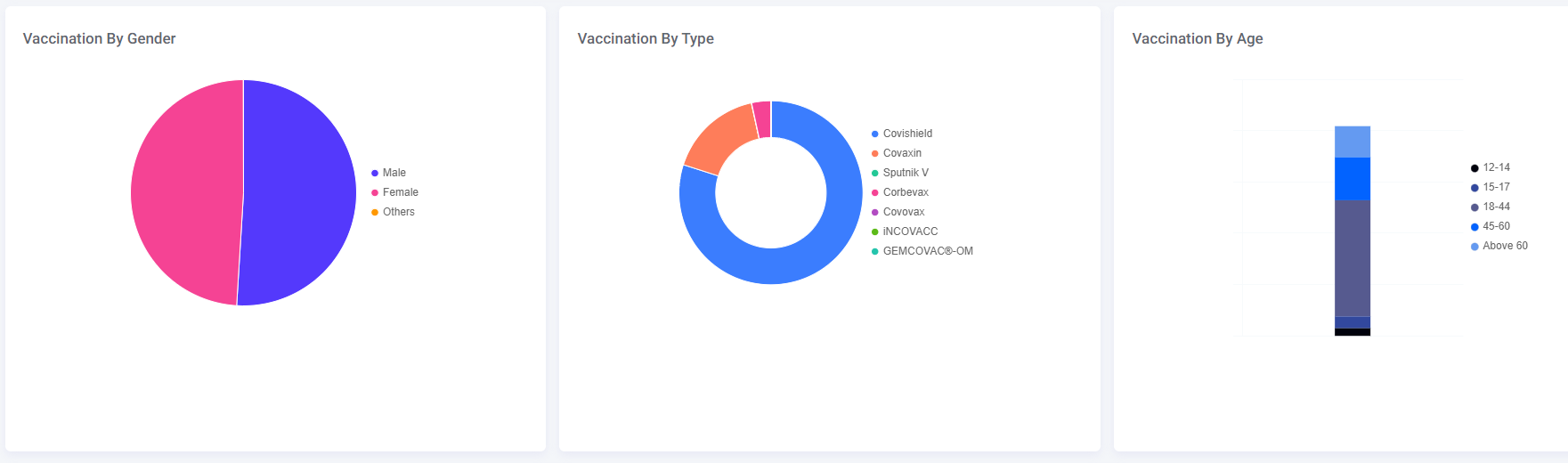
Photo Credit: https://dashboard.cowin.gov.in/
The overwhelming majority of the population received the Covishield vaccine, while a small section got the Corbevac jab.
Serum Institute On Covishield
The SII told NDTV that all its product packaging had “disclosed all rare to very rare side-effects”, including TTS, and pointed out the vaccine had been “instrumental” in saving lives worldwide.
READ | “We Disclosed All Rare Side-Effects”: Covid Vaccine Maker On Safety Row
The company also said it discontinued Covishield manufacture from 2021 due to “the emergence of new mutant variant strains”, which had led to decreased demand for previous vaccines”.
NDTV is now available on WhatsApp channels. Click on the link to get all the latest updates from NDTV on your chat.




